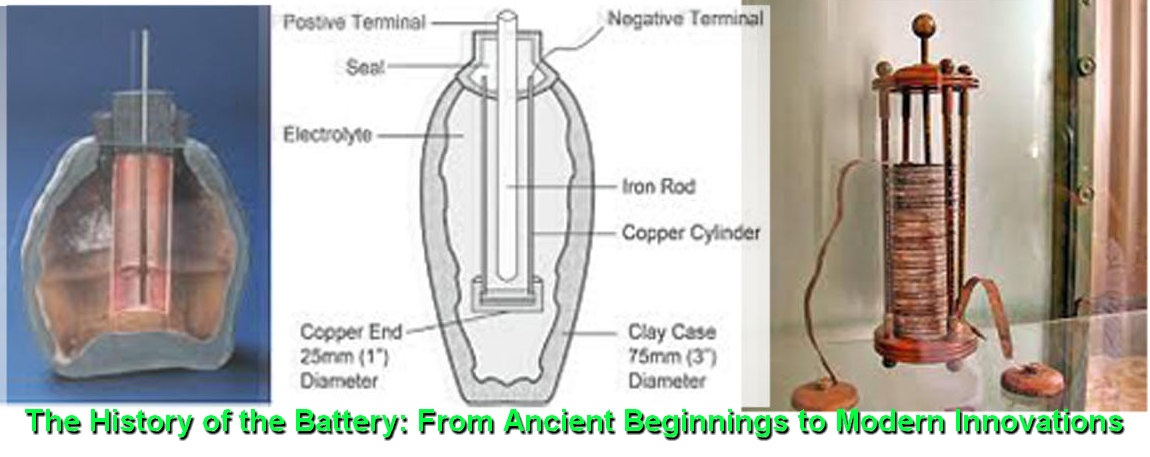
The History of the Battery: From Ancient Beginnings to Modern Innovations
The History of the Battery is a cornerstone of modern life, powering everything from smartphones to electric vehicles. Yet, its story is as fascinating as the technology itself, spanning thousands of years of human ingenuity and innovation. This article traces the history of the battery, exploring its ancient origins, groundbreaking scientific discoveries, and its pivotal role in shaping the modern world.
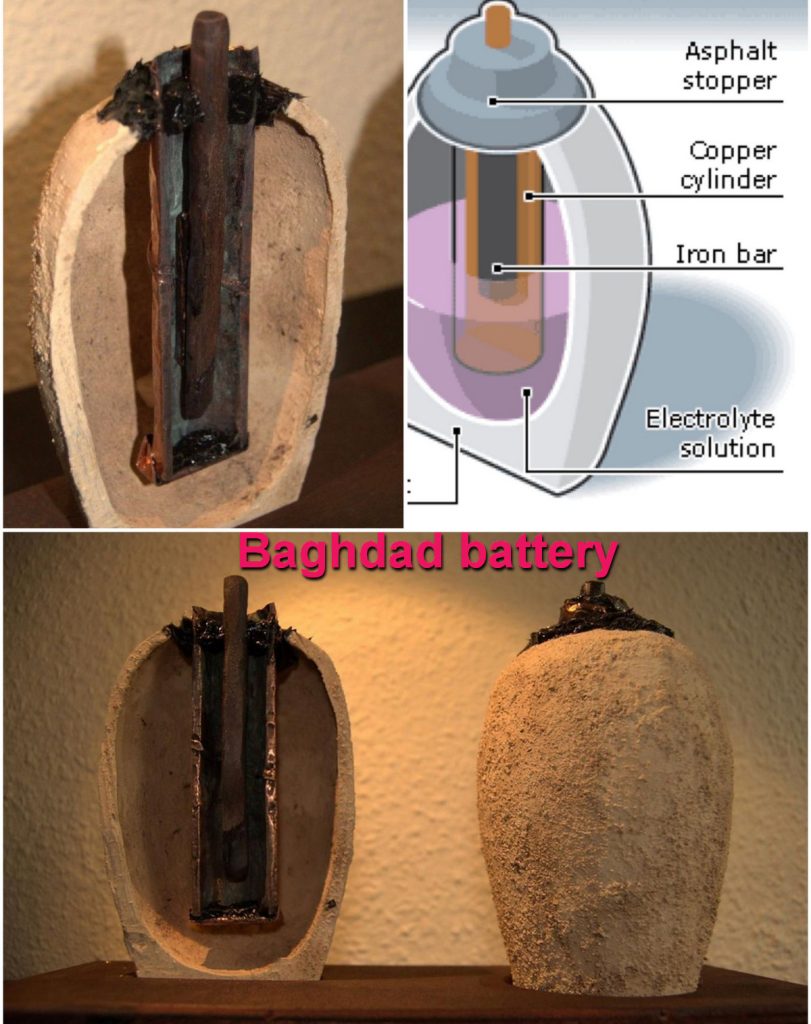
Ancient Beginnings: The Baghdad Battery
The earliest known precursor to the modern battery dates back to around 250 BCE. Archaeologists discovered a peculiar artifact near Baghdad in the 1930s, now famously known as the “Baghdad Battery.” This clay jar, containing a copper cylinder and an iron rod, is believed to have been capable of generating a small electric current when filled with an acidic liquid such as vinegar or lemon juice. While its exact purpose remains a topic of debate, theories suggest it may have been used for electroplating or religious rituals.
Though rudimentary by today’s standards, the Baghdad Battery hints at humanity’s early experimentation with electricity, laying the groundwork for future advancements.
The Birth of Modern Batteries: Alessandro Volta’s Pile
The true birth of the modern battery came in 1800 with Alessandro Volta, an Italian physicist and chemist. Volta’s invention, known as the “Voltaic Pile,” consisted of alternating layers of zinc and copper discs separated by cloth soaked in saltwater or acid. This configuration generated a steady electrical current, marking the first time electricity could be produced in a controlled and continuous manner.
Volta’s breakthrough was revolutionary. It not only demonstrated that electricity could be generated chemically but also provided scientists with a reliable source of electric power for experiments. This invention earned Volta widespread acclaim, and the unit of electric potential, the volt, was named in his honor.
19th Century Advancements: From Daniell to Leclanche
The 19th century witnessed rapid advancements in battery technology. In 1836, John Frederic Daniell, a British chemist, introduced the Daniell Cell. This battery used copper and zinc electrodes submerged in separate solutions, connected by a porous barrier. The Daniell Cell was more stable and produced a higher voltage than the Voltaic Pile, making it a popular choice for early telegraph systems.
Later, in 1866, Georges Leclanché developed the Leclanché Cell, which used a zinc anode, a manganese dioxide cathode, and an ammonium chloride electrolyte. This design was the precursor to the modern dry cell battery, as it eliminated the need for liquid electrolytes, making it more portable and practical for everyday use.
The Birth of Rechargeable Batteries
The invention of rechargeable batteries marked another milestone in the history of energy storage. In 1859, French physicist Gaston Planté invented the lead-acid battery, the first rechargeable battery. Planté’s design featured lead plates immersed in sulfuric acid. When charged, the battery could store and deliver significant amounts of energy, making it suitable for industrial applications and, later, automobiles.
The lead-acid battery’s ability to be recharged multiple times revolutionized energy storage, paving the way for technologies such as electric vehicles and backup power systems.

The Alkaline Era: Edison and Beyond
At the turn of the 20th century, Thomas Edison developed the nickel-iron battery, another rechargeable system. While less energy-dense than lead-acid batteries, nickel-iron batteries were durable and capable of withstanding frequent charging and discharging cycles. Edison envisioned them as a power source for electric vehicles, but their relatively high cost and low efficiency limited widespread adoption.
In 1955, Lewis Urry, a Canadian engineer, invented the modern alkaline battery while working for Eveready Battery Company. Urry’s design, which used zinc and manganese dioxide, offered longer life and improved performance compared to earlier dry cells. Alkaline batteries quickly became the standard for household electronics and remain widely used today.
The Lithium Revolution
The development of lithium-based batteries in the late 20th century marked a turning point in battery technology. Lithium, being the lightest metal with a high electrochemical potential, offered significant advantages in energy density and weight.
In 1970, Stanley Whittingham developed the first lithium-ion battery prototype, using titanium disulfide and lithium metal. However, safety concerns and material limitations hindered its commercialization. It wasn’t until the 1980s that John Good enough, Rachid Yazami, and Akira Yoshino made critical advancements, leading to the development of the modern lithium-ion battery.
Goodenough’s use of lithium cobalt oxide as the cathode material increased energy density significantly. Yazami’s contribution involved the use of graphite as the anode, which improved safety and efficiency. Yoshino later combined these innovations into a practical, rechargeable lithium-ion battery, which Sony commercialized in 1991.
Lithium-ion batteries transformed the world of portable electronics, enabling the development of smartphones, laptops, and eventually electric vehicles. Their high energy density and rechargeability have made them the cornerstone of modern energy storage.
Batteries in the 21st Century
As the demand for renewable energy and electric vehicles has grown, so too has the need for advanced battery technologies. The 21st century has seen significant progress in energy storage solutions, including the development of:
- Solid-State Batteries: These batteries replace liquid electrolytes with solid materials, offering improved safety, energy density, and longevity. Solid-state batteries are seen as a potential game-changer for electric vehicles and portable electronics.
- Lithium-Sulfur Batteries: With a higher theoretical energy density than lithium-ion batteries, lithium-sulfur technology holds promise for applications requiring lightweight, high-capacity storage.
- Flow Batteries: Designed for large-scale energy storage, flow batteries use liquid electrolytes stored in external tanks. This design allows for scalability and long cycle life, making them ideal for integrating renewable energy sources into the grid.
- Sodium-Ion Batteries: Sodium-ion technology offers a cost-effective and sustainable alternative to lithium-ion batteries, leveraging abundant sodium resources. While still in development, these batteries show promise for stationary energy storage.
The Future of Battery Technology
The quest for better batteries continues, driven by the need to address climate change and transition to a sustainable energy future. Researchers are exploring novel materials, such as silicon anodes and graphene, to enhance battery performance. Meanwhile, advances in artificial intelligence and nanotechnology are accelerating the discovery of new battery chemistries and designs.
One of the most exciting areas of research involves recycling and sustainability. Efforts to develop efficient recycling methods aim to recover valuable materials from used batteries, reducing environmental impact and reliance on finite resources.
Culmination
From the enigmatic Baghdad Battery to the cutting-edge lithium-ion technologies of today, the history of the battery is a testament to human ingenuity and perseverance. Each innovation has built upon the work of earlier pioneers, transforming the way we live, work, and interact with the world.
As we look to the future, batteries will continue to play a vital role in addressing global challenges, from enabling the widespread adoption of renewable energy to revolutionizing transportation. The journey of the battery is far from over, and the next chapter promises to be just as groundbreaking as the last.